September/October 2014
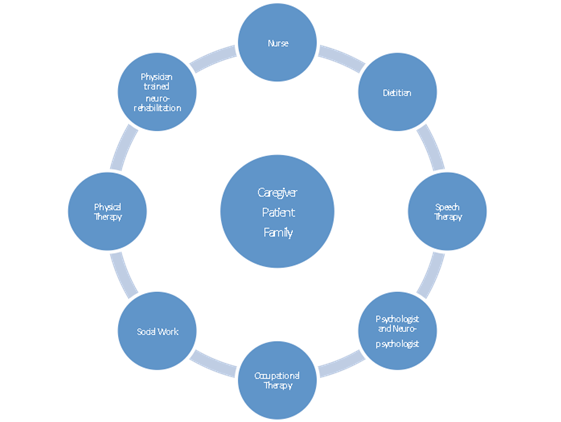
Stroke Rehabilitation: Strengthening Synapses to Achieve Optimal Outcomes Successful stroke rehabilitation requires individualized treatment focusing on reorganization in the recovering brain, a multidisciplinary rehabilitation approach, goal setting, and community therapy resources. Stroke is one of the leading causes of death in industrialized countries, and complications arising from stroke present a major personal and economic burden. Studies show that up to 75% of stroke survivors have disabilities that ultimately affect their employability and livelihood.1 Every year more than 750,000 people in the United States suffer a stroke, and there are more than 6 million stroke survivors in the United States alone.2 As a result, millions of people in the United States are in need of rehabilitation to help them return to function, independence, social activities, and community participation. Limitations on activity and participation3 can be classified as affecting activities of daily living (ADLs) or basic self-care activities such as dressing, bathing, or toileting.4 Such limitations may also affect functions or instrumental ADLs, such as housework, meal preparation, shopping, using the telephone, and other tasks that allow independent community living.5 It is important to understand that visible disabilities such as paralysis are not the only factors that limit people after stroke. Hidden disabilities such as depression, problems with cognition, memory, and communication; and functional vision problems such as visual-spatial deficits may also be disabling, even though they are not as easily identified on casual observation. The recovery period following a stroke may range from weeks to months. Because recovery may frequently continue even after one year,6 it is very important at the time of stroke for all members of the medical team to introduce the rehabilitation process to stroke survivors and their families. Stroke survivors and their families benefit from support to prepare themselves for the care of both visible and hidden disabilities in a plan of care that transitions from the hospital to the home, outpatient rehabilitation, and beyond, with therapeutic options at each step.7 This article discusses several rehabilitation approaches, or therapies, that can be used following stroke to improve daily life function. The authors will outline the way in which each therapy is thought to work, which candidates may benefit, and the important characteristics of each technique. The article describes two categories of therapies: those intended to speed stroke recovery by generally enhancing brain activity, or stimulation-based approaches, and therapies intended to focus and target specific task practice. Clinicians may be unfamiliar with some of the newer therapies. The authors also touch on the advantages of using these approaches, as compared with more conventional or traditional techniques. In discussing new treatments, the authors will also review critical components of successful stroke recovery: reorganization in the recovering brain, a multidisciplinary approach to rehabilitation, goal setting, and community therapy resources. Providing access to rehabilitation and encouraging stroke survivors and their families to take advantage of therapy will increase the odds of success in transitioning home after hospitalization. When health care providers inform the survivor and family about the rehabilitation process, it can help prepare the stroke survivor to take the steps necessary to return to former social and occupational roles. Stimulation-Based Approaches It is now known that neurons’ growth from neural progenitors does occur in some parts of the brain throughout life9 and that our functional brain circuitry remains capable of change after injury. As demonstrated in numerous studies since the classical work of Harlow and Harlow,10 rich environmental stimulation is needed to support healthy brain development, and the same kind of environmental resources are needed for poststroke recovery of brain networks supporting movement, communication, memory, and functional three-dimensional body movements. Two emerging physiological brain stimulation approaches that have attracted considerable attention are noninvasive methods of applying electrical and magnetic energy directly to the cortex.11 Transcranial magnetic stimulation (TMS) uses electromagnetic technology to create an electrical current that can depolarize neurons. A brief, strong magnetic field created by a coil-circulated electrical current penetrates the skull when the stimulator applicator is held at the proper orientation near the head. First introduced in 1985, TMS has been used for both diagnostic and therapeutic indications in a range of neurologic and psychiatric conditions.12 Repetitive application of electrical stimulation via TMS (rTMS) is approved as a clinical treatment for depression, when applied to the prefrontal cortex of the left brain. Because the excitatory or inhibitory effect of TMS on the cortex may persist with a repeated series of stimulation administered via rTMS, the hope is that clinical trials will indicate that poststroke hemiparesis and other neurological conditions may be treatable with this technique. At this point, only adult candidates with major depressive disorder who have failed a prior trial of antidepressant medication are eligible for rTMS treatment. A history of epilepsy, stroke, tumors, or infectious lesions of the brain, sleep disorders, alcoholism, pregnancy, or severe heart disease, and other neurological disorders are contraindications. However, it is notable that some research studies report stroke recovery associated with TMS applied to the healthy hemisphere, where there is a decreased risk of treatment. Although TMS provides stimulation over a relatively large cerebral region, the region over which stimulation is applied differs depending on the indication being examined (eg, clinical trials examining rTMS for hemiparesis may stimulate motor cortex). Transcranial direct current stimulation (tDCS) uses electrical stimulation to enhance cortical excitability. Saline-soaked pads placed on the scalp apply a very weak current (about two milliamperes) in 10- to 30-minute sessions to modify rather than generate neuronal activity in cortical neurons by generating an electrical field that fosters neural network interaction. Although tDCS is being investigated to improve paralysis, depression, and pain in neurological disorders,11 it is a research intervention only, without FDA-approved clinical indications. Further clinical trials are needed to determine the benefit of tDCS to the ADLs, and whether these benefits are short-lived or meaningful over longer time periods for recovery. Therapies Based on Focused Intensive Practice Perhaps the best example of intensive, experience-based, repetitive training is motor rehabilitation of a paretic limb in constraint-induced movement therapy (CIMT), first used in stroke survivors in the 1980s based on the observation of “learned nonuse” in monkeys with a deafferented limb but intact motor capability.14 Immobilizing the unaffected limb restored competent, symmetric movements in the deafferented limb; intensive use of the impaired limb supported return of its functional use. CIMT is a therapeutic strategy developed to overcome learned nonuse of the paretic limb, and although applied primarily to stroke survivors, it has also been used in other neurological disorders.15 The unaffected arm is restrained with a sling or glove, and the patient is involved in intensive, repetitive training to maximize the use of the paretic arm through the performance of functionally oriented activities. Some studies supported actual structural changes in the motor network, presumably occurring as a result of this treatment.16 Candidates for CIMT should have some degree of extension in the paretic extremity, conventionally 10 to 15 degrees of extension at the elbow, wrist, and fingers. Candidates should also have the cognitive skills and personal maturity to make a commitment to the therapy, enforced via a behavioral contract in a “transfer package.”17 A major advantage of the CIMT approach is that it is low risk and feasible in many settings. The main cost is therapist time, which, unfortunately, many third-party payers may not reimburse. There are several differences between the CIMT approach and typical outpatient therapy for stroke survivors. First, typical outpatient physical, occupational, and speech therapy programs involve two to three sessions of about 45 minutes per week, for a period of a few weeks to months, depending on patient needs. However, a CIMT program involves more frequent, long sessions over a shorter period. A CIMT program can run for three to six hours each day for two weeks, with individualized outcome assessment. CIMT can be effective even years after the stroke occurs, but typically stroke survivors are not prescribed outpatient therapy beyond the first six months after the stroke. The important transfer package or behavioral contract involves the need to make a conscious agreement to work toward therapy progress as a joint goal through this contract between the therapist and patient, increasing its patient-centered properties. Constraint-induced language therapy (CILT) is a novel approach that applies the principles of constraint-induced motor rehabilitation to treat aphasia. Patients with aphasia receiving CILT receive longer, more frequent sessions as with CIMT. However, the constraint applied is restriction to the ability to use compensatory communication strategies, such as gesturing and drawing. In some settings, this constraint is a physical barrier to block gestures; in other settings, therapists as conversation partners simply withhold response to communicative output that is nonverbal. Intensive practice occurs in one-to-one exchanges or in group exercises such as a modified card game. The goal is to implement constraints to facilitate the patient’s engagement in massed practice of language functions. As above, CILT is believed to be effective because it supports beneficial remodeling of brain language networks; participants can experience improved language performance within 10 days of implementation.18 Although most people receiving CILT in research or clinical settings have nonfluent aphasia syndromes, it is not yet clear which people with aphasia respond best to the technique. It is widely agreed, however, that clients must have sufficient pragmatic comprehension to take part voluntarily in the constraint aspect of treatment. This fits the concept of person-centered care as a central part of constraint-induced therapies, and the importance even in CILT of a behavioral contract or therapy agreement. Focused, Intensive Therapy for Right Brain Stroke Recovery Patients repeatedly point to targets or perform continuous manual tasks while the view of their own arm movements is partially blocked. A major advantage to using PAT is its action on low-level, implicit brain systems—clients make more leftward movements after right brain stroke, but this is not because they are told to do so but because their movement system automatically readjusts. Since patients with right brain stroke frequently overestimate their own abilities,20 this lightens the burden for a patient who already has difficulty knowing when to use strategies and when they are not needed. The ideal patient for PAT has spatial neglect as defined by a standard assessment,21 can sit up, and has vision in at least one eye that is adequate to point to a target. Although the currently recommended treatment period, as with CIMT, is only 10 consecutive sessions, longer periods of treatment may also be helpful for patients who continue to show evidence of spatial neglect affecting their ability to transfer, use a wheelchair, eat, or perform higher-level tasks such as working or sports. Challenges to Optimal Stroke Rehabilitation Introducing the Rehabilitation Plan and Expectations Stroke survivors and families should be familiar with the rehabilitation roadmap and the resources available for the chronic stage of recovery, extending from the acute inpatient hospital to inpatient rehabilitation, long term acute care or skilled nursing care, home nursing, outpatient care, and rehabilitation. Too frequently, survivors and families assume that rehabilitation options end at one month, three months, or six months; they may have left the hospital unprepared to self-advocate and take an active role in identifying therapy resources that might be available at nearby colleges, research programs, peer support centers, or other organizations. An informed stroke survivor and family can partner with the family physician to find the best options available at each stroke recovery stage. Planning During Inpatient Care Organizing multidisciplinary Therapy and Focused Treatment
Final Thoughts — Mosunmola Oyawusi, MD, is a postdoctoral fellow in stroke rehabilitation research at the Kessler Foundation and stroke rehabilitation at the Kessler Institute for Rehabilitation. — Uri Adler, MD, is the director of stroke rehabilitation services at the Kessler Institute for Rehabilitation at the Saddle Brook campus. He is an assistant professor of physical medicine and rehabilitation at Rutgers-New Jersey Medical School. — A.M. Barrett, MD, is the director of stroke rehabilitation research at the Kessler Foundation and chief of neurorehabilitation program innovation at the Kessler Institute for Rehabilitation. She is a professor of physical medicine and rehabilitation at Rutgers-New Jersey Medical School and past president of the American Society for Neurorehabilitation. References 2. Go AS, Mozaffanan D, Roger UL, et al. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation 2014; 129(3):e28-e292. 3. Jette AM. Toward a common language for function, disability and health. Phys Ther. 2006;86(5):726-734. 4. Wiener JM, Hanley RJ, Clark R, Van Nostrand JF. Measuring the activities of daily living: comparisons across national surveys. J Gerontol. 1990;45(6):S229-S237. 5. Bookman A, Harrington M, Pass L, Reisner E. The Family Caregiver Handbook: Finding Elder Care Resources in Masssachusetts. Cambridge, MA: Massachusetts Institute of Technology Press, 2007. 6. National Institute of Neurological Disorders and Stroke. Post-stroke rehabilitation fact sheet. NIH Publication No. 08-4846, October 2008. http://www.ninds.nih.gov./disorders/stroke/poststrokerehab.htm. Accessed August 23, 2014. 7. Bagherpour R, Dykstra DD, Barrett AM, Luft AR, Divani AA. A comprehensive neurorehabilitation program should be an integral part of a comprehensive stroke center. Front Neurol. 2014;5:57. 8. Rosenzweig MR, Krech D, Bennett EL, Diamond MC. Effects of environmental complexity and training on brain chemistry and anatomy: a replication and extension. J Comp Physiol Psychol. 1962;55(4):429-437. 9. Lledo PM, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 2006;7(3):179-193. 10. Harlow HF, Harlow MK. The effect of rearing conditions on behavior. Bull Menninger Clin. 1962;26:213–224. 11. Fregni F, Pascual-Leone A. Technology insight: noninvasive brain stimulation in neurology—perspectives on the therapeutic potential of rTMS and tDCS. Nat Clin Pract Neurol. 2007;3(7):383-393. 12. Rossini PM, Rossi S. Transcranial magnetic stimulation: diagnostic, therapeutic, and research potential. Neurology. 2007;68(7):484-488. 13. Cramer SC, Duncan PL, Barrett AM (Co-Chairs). Final report of the Stroke Progress Review Group: Recovery and Rehabilitation. http://www.ninds.nih.gov/about_ninds/groups/stroke_prg/2012-stroke-prg-full-report.htm - RR. January 2012. Accessed August 23, 2014. 14. Huang WC, Chen YJ, Chien CL, Kashima H, Lin KC. Constraint-induced movement therapy as a paradigm of translational research in neurorehabilitation: reviews and prospects. Am J Transl Res. 2011;3(1):48-60. 15. Geerdink Y, Aarts P, Geurts AC. Motor learning curve and long-term effectiveness of modified constraint-induced movement therapy in children with unilateral cerebral palsy: a randomized controlled trial. Res Dev Disabil. 2013;34(3):923-931. 16. Gauthier LV, Taub E, Perkins C, Ortmann M, Mark VW, Uswatte G. Remodeling the brain: plastic structural brain changes produced by different motor therapies after stroke. Stroke. 2008;39(5):1520-1525. 17. Taub E. The behavior-analytic origins of constraint-induced movement therapy: an example of behavioral neurorehabilitation. Behav Anal. 2012;35(2):155-178. 18. Meinzer M, Rodriguez AD, Gonzalez Rothi LJ. First decade of research on constrained-induced treatment approaches for aphasia rehabilitation. Arch Phys Med Rehabil. 2012;93(1):S35-S45. 19. Barrett AM, Goedert KM, Basso JC. Prism adaptation for spatial neglect after stroke: translational practice gaps. Nat Rev Neurol. 2012;8(10):567-577. 20. Barrett AM. Rose-colored answers: neuropsychological deficits and patient-reported outcomes after stroke. Behav Neurol. 2010;22(1-2):17-23. 21. Chen P, Hreha K, Fortis P, Goedert KM, Barrett AM. Functional assessment of spatial neglect: a review of the Catherine Bergego Scale and an introduction of the Kessler Foundation Neglect Assessment Process. Top Stroke Rehabil. 2012;19(5):423-435. 22. Centers for Disease Control and Prevention. Outpatient rehabilitation among stroke survivors—21 states and the District of Columbia, 2005. MMWR Morb and Mortal Wkly Rep. 2007;56(20):504-507. 23. Riestra AR, Barrett AM. Rehabilitation of spatial neglect. Handb Clin Neurol. 2013;110:347-355. |