May/June 2017
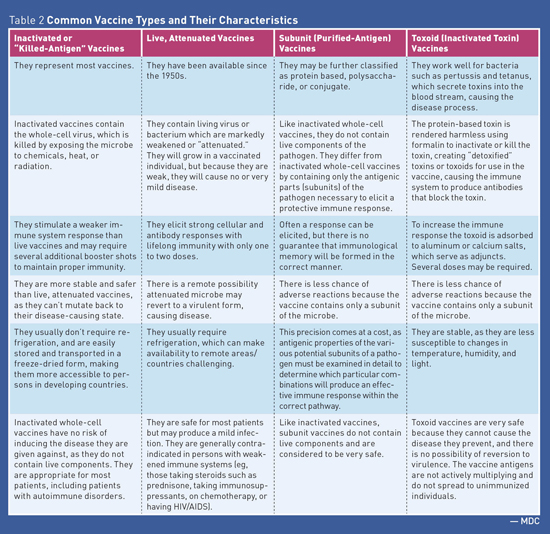
Medication Monitor: Vaccines and Autoimmune Disease Vaccine Safety Federal law requires that Vaccine Information Statements explaining vaccine benefits and risks be provided when certain vaccinations are administered (including before each dose). Vaccine Information Statements are available in Spanish and many other languages. In addition, more detailed information describing the benefits and risks of vaccines is available in the Licensed Vaccine List from the FDA.1 False Reports The protective value achieved from receiving vaccines is well established and is based on sound epidemiologic data both for the general population as well as for those individuals with AD. While there is a rare possibility that vaccines may induce or exacerbate AD, the basis for making widespread claims is highly speculative and is based on isolated case reports.4 Autoimmune Disease and Infection Risk Immunosuppressive Treatments • corticosteroids or "steroids" (eg, prednisone); • disease-modifying antirheumatic drugs (DMARDS) such as methotrexate, leflunomide, azathioprine, cyclosporine, and mercaptopurine; and • biologic agents also referred to as "biologics" or targeted immune modulators such as antitumor necrosis factor agents (eg, etanercept, adalimumab), belimumab, and rituximab. Biologic agents are distinguished from other immunosuppressives in that they are produced by living systems, such as bacteria or plant or animal cells. As soon as an individual begins using biologics to modify the immune system, his or her immune system's ability to fight off and prevent infection is severely suppressed.6 Biologics and other immunosuppressive agents can increase the risk of infections, including the following: • bacterial sepsis; • Mycobacterium tuberculosis infection recrudescence; • fungal infection/reactivation; and • viral reactivation, eg, varicella-zoster virus (shingles) or worsening of a chronic viral infections such as hepatitis B infection. Vaccine Types See Table 1 and Table 2 for additional information about vaccine types.
Vaccine Use in Individuals With AD7 This may be due to education about the differences in types of vaccines, inadequate communication between primary care physicians and specialists, patient misconceptions about safety, and fear of flares, which are sudden and severe onset of symptoms. In general, while flares are possible, they are rare when guideline recommendations are followed. Inactivated Vaccines7 Persons with AD who become infected with influenza, pneumonia, or hepatitis B are at increased risk of more severe disease and increased mortality/morbidity than immunocompetent persons. Therefore, persons with AD are strongly encouraged to receive these vaccinations. Influenza (intramuscular injection), pneumococcal, and hepatitis B vaccines are inactivated vaccines and are safe and generally sufficiently immunogenic in patients with AD. • Influenza (intramuscular): For patients with AD, a yearly intramuscular (which is inactivated) influenza vaccination is recommended. Patients aged 65 and older should receive the high-dose vaccine, which has been shown to be more effective in this age group. • Pneumococcal: The CDC recommends vaccination for all immunosuppressed adults naive to pneumococcal vaccinations. The CDC recommends vaccination with PCV13 (pneumococcal conjugate vaccine) followed by PPSV23 (pneumococcal polysaccharide vaccine) in eight weeks to one year later. • Hepatitis B vaccine (HBV): For patients with AD, the severity of hepatitis B infection is more severe with increased morbidity rates than for persons who are immunocompetent. Therefore, hepatitis B vaccination is of particular importance in patients with AD, as biologic therapy can increase the risk of reactivation in those infected. Before initiating biologic therapy, HBV serology should be checked in all patients. Some experts recommend initiating hepatitis B vaccination at the time an AD is diagnosed. Tetanus, diphtheria, and acellular pertussis vaccines are inactivated vaccines and, as in the general population, the immunization status of patients with AD should be checked and vaccination considered for tetanus, diphtheria, and pertussis vaccination. • Tetanus and diphtheria: Tetanus and diphtheria vaccinations are recommended once every 10 years. • Pertussis: The pertussis vaccine helps protect against whooping cough, which is highly contagious, causing prolonged distinct coughing. Pertussis remains incompletely controlled in the United States and is considered an epidemic worldwide. The pertussis vaccine is recommended in childhood with one booster in adulthood. Live Vaccines7 Herpes Zoster Vaccine (ZOS) ZOS can be given to patients with AD even if they are receiving the following: • short-term, low-dose, or local (eg, topical or nonsystemic administration) corticosteroid therapy; and • low-dose treatment defined as using methotrexate (<0.4 mg/kg/week), azathioprine (<3 mg/kg/day), or 6-mercaptopurine (<1.5 mg/kg/day) for the treatment of rheumatoid arthritis, psoriasis, polymyositis, sarcoidosis, inflammatory bowel disease, or other conditions. ZOS does not need to be delayed in these persons, and it can be administered to contacts of patients with AD and other chronic diseases or altered immunocompetence. Additional Guidance for Live Vaccines7 Persons with AD who are being treated with immunosuppressive therapy must be regarded as immunocompromised individuals, although the extent to which immune competence is impaired depends on the type and dose of medication used, as well as the duration of therapy. Administer LAVs no earlier than three to four weeks before the start or restart of immunosuppressive therapy to ensure that virus replication has ended before impairing a patient's immune competence. In order to safely administer a LAV to a person with AD with respect to the exact time period after which immunosuppressive therapy has been discontinued, it depends on the type, dose, and duration of the therapy; however, a safe rule of thumb is to allow a period of three months for the immune status to be completely restored, except for corticosteroid therapy, where a waiting period of one month is thought to be sufficient. — Mark D. Coggins, PharmD, CGP, FASCP, is vice president of pharmacy services for Diversicare, which operates skilled nursing centers in 10 states. He was nationally recognized by the Commission for Certification in Geriatric Pharmacy with the 2010 Excellence in Geriatric Pharmacy Practice Award. References 2. Williams WW, Lu PS, O'Halloran A, et al. Surveillance of vaccination coverage among adult populations — United States, 2014. MMWR Surveill Summ. 2016;65(1):1-36. 3. Vaccines do not cause autism. Centers for Disease Control and Prevention website. https://www.cdc.gov/vaccinesafety/concerns/autism.html. Updated November 23, 2015. 4. Murdaca G, Orsi A, Spanò F, et al. Influenza and pneumococcal vaccinations of patients with systemic lupus erythematosus: current views upon safety and immunogenicity. Autoimmun Rev. 2014;13(2):75-84. 5. Autoimmune disease. Healthline website. http://www.healthline.com/health/autoimmune-disorders?m=0#treatment6 6. Brause B. Vaccinations and lupus: an update of what you should know. Hospital for Special Surgery website. https://www.hss.edu/conditions_vaccinations-lupus-update.asp. Published June 17, 2014. 7. Rahier JF, Moutschen M, Van Gompel A, et al. Vaccinations in patients with immune-mediated inflammatory diseases. Rheumatology (Oxford). 2010;49(10):1815-1827. |