September/October 2016
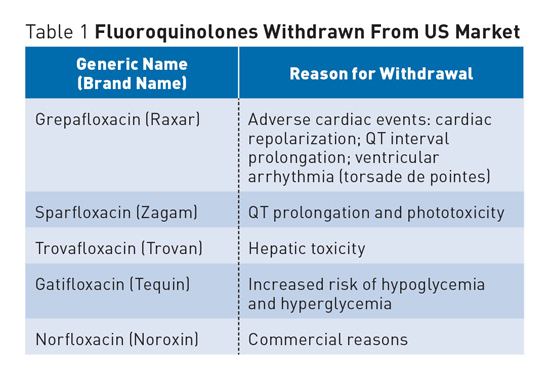
Fluoroquinolone Antibiotic Risks A number of FDA medication advisories have been issued in recent years as a result of increased awareness of serious side effects associated with fluoroquinolones, a class of broad-spectrum, systemic antibacterial agents that have been widely used for a number of infections, including respiratory and urinary tract infections. In addition to increased warnings, a number of fluoroquinolone antibiotics have been withdrawn from the US market and other countries due to various concerns (see Table 1).
FDA Advisories and Black Box Warnings The FDA noted that safety reviews have shown that fluoroquinolones, when used systemically (ie, oral or injectable), are associated with disabling and potentially permanent serious side effects that can occur together. These side effects can involve the tendons, muscles, joints, nerves, and central nervous system. As a result, the FDA required manufacturers to update fluoroquinolone drug labeling and their associated Medication Guides to reflect this new safety information.1,2 Patients should be counseled about the potential serious side effects associated with the use of fluoroquinolones to help minimize potential harm. Educate patients on the signs and symptoms of serious side effects, which include tendon, joint, and muscle pain, a "pins and needles" tingling or pricking sensation, confusion, and hallucinations. Instruct patients to contact their health care professionals immediately if they experience any serious side effects while taking any of the fluoroquinolone agents.1,2 The FDA also recommends that prescribers stop systemic fluoroquinolone treatment immediately if a patient reports serious side effects, and to switch to a nonfluoroquinolone antibacterial drug to complete the patient's treatment course.2 Peripheral Neuropathy2 The risk of peripheral neuropathy is associated only with systemic fluoroquinolones taken by mouth or by injection. Topical formulations of fluoroquinolones, applied to the ears or eyes, are not known to be associated with this risk. Tendinitis and Tendon Rupture Black Box Warning3 Tendinitis and tendon rupture most frequently involve the Achilles; surgical repair is sometimes required. Tendinitis and tendon rupture have also been reported to occur in the shoulder (specifically, the rotator cuff), the hand, the biceps, and the thumb. Tendon rupture can occur during or after completion of fluoroquinolone use; cases have been reported to have occurred up to several months after completion of therapy. Inform patients that tendons are the areas that connect muscles to joints and that the Achilles tendon is at the back of the ankle. Health care professionals and patients should recognize pain, swelling, inflammation, and tears of tendons including the Achilles, shoulder, hand, or other tendons as possible side effects of fluoroquinolone treatment. The risk for tendon problems is higher in patients who are over the age of 60, taking steroids (corticosteroids), and who have undergone kidney, heart, or lung transplant. Other reasons for tendon ruptures and increased risk include physical activity or exercise, kidney failure, and prior tendon problems, such as with those occurring with rheumatoid arthritis. Patients receiving fluoroquinolones should notify their health care providers immediately at the first signs or symptoms of pain, swelling, or inflammation in a tendon area, and should avoid exercise and use of the affected area. The fluoroquinolone medication should be stopped until it is determined whether it results from tendinitis or a tendon rupture. Signs or symptoms of tendon rupture include the following:
Other Side Effects and Concerns QT Prolongation Retinal Detachment Hypoglycemia and Hyperglycemia Dosing Adjustments For patients with impaired renal function, the following adjustments should be made: Antimicrobial Stewardship — Mark D. Coggins, PharmD, CGP, FASCP, is senior director of pharmacy services for skilled nursing centers operated by Diversicare in nine states, and is a director on the board of the American Society of Consultant Pharmacists. He was nationally recognized by the Commission for Certification in Geriatric Pharmacy with the 2010 Excellence in Geriatric Pharmacy Practice Award. References 2. US Food and Drug Administration. FDA drug safety communication: FDA requires label changes to warn of risk for possibly permanent nerve damage from antibacterial fluoroquinolone drugs taken by mouth or by injection. http://www.fda.gov/downloads/Drugs/DrugSafety/UCM365078.pdf. Published August 15, 2013. Accessed August 1, 2013. 3. Information for healthcare professionals: fluoroquinolone antimicrobial drugs [ciprofloxacin (marketed as Cipro and generic ciprofloxacin), ciprofloxacin extended-release (marketed as Cipro XR and Proquin XR), gemifloxacin (marketed as Factive), levofloxacin (marketed as Levaquin), moxifloxacin (marketed as Avelox), norfloxacin (marketed as Noroxin), and ofloxacin (marketed as Floxin)]. US Food and Drug Administration website. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ 4. Johanesen PA, Mackin KE, Hutton ML, et al. Disruption of the gut microbiome: clostridium difficile infection and the threat of antibiotic resistance. Genes. 2015;6(4):1347-1360. 5. Kang J, Wang L, Chen XL, Triggle DJ, Rampe D. Interactions of a series of fluoroquinolone antibacterial drugs with the human cardiac K+ channel HERG. Mol Pharmacol. 2001;59(1):122-126. 6. QTdrugs list. CredibleMeds website. www.Crediblemeds.org. Updated August 10, 2016. 7. Etminan M, Forooghian F, Brophy JM, Bird ST, Maberley D. Oral fluoroquinolones and the risk of retinal detachment. JAMA. 2012;307(13):1414-1419. 8. Hypoglycemia and hyperglycemia with fluroroquinolones. Med Lett Drugs Ther. 2003;45(1162):64. |