Fall 2025
Fall 2025 Issue Balancing Issues Age-related balancing issues are costly and debilitating. Declining balance and mobility, or presbystasis,1 is one of the most visible, debilitating, and costly signs of aging, undermining health, independence, and quality of life. Dysfunction of the vestibular balance organs in the inner ear, or presbyvestibulopathy,2 is a central cause of mobility loss and can trigger a spiraling decline that also impacts cognitive function3 and psychological well-being.4 Despite an economic burden exceeding $280 billion annually in the United States,5,6 no easy-to-use tools are available to diagnose age-related vestibular changes, and experimental high-risk surgical vestibular implants7 remain the only available restorative treatment option. Innovations in wearable diagnostics8,9 and noninvasive bioelectronic treatments10-13 have been successfully combined to activate long-lasting neuroplastic restoration of vestibular function, balance, and mobility in older adults aged 51 to 98 years. Results from pilot deployments in clinics, senior communities, and home care settings demonstrate that these accessible innovations enable minimally trained clinical staff and senior care providers to deliver dramatic levels of balance restoration safely and effectively outside traditional clinical facilities. Aging Biomarkers vs Restorative Interventions Recent innovations have addressed two key challenges that impede access to widely available diagnosis and treatment for declining mobility. A new class of digital biomarkers, utilizing a head-mounted wearable sensor and a simple two-minute balance test, quantifies the progressive decline of multiple physiological systems, including the vestibular system.8 These novel biomarkers serve as the basis for a clinically validated, noninvasive bioelectronic vestibular stimulation device and a six-week treatment protocol that together deliver extensive and persistent neuroplastic restoration of balance and mobility in study populations from 51 to 98 years of age.10 Balance Physiology and Aging • The vestibular system detects head movement and spatial orientation. • Vision provides an external reference for orientation. • Proprioception senses joint and muscle position. The CNS integrates these inputs to generate motor control output signals, which are then transmitted to the musculoskeletal system via the peripheral nervous system. As people age, the performance of all these systems declines.19 Sensory reweighting (SR) is a key element of CNS processing related to motor control.20 A feature of brain plasticity, SR adjusts the brain’s reliance on sensory inputs (Figure 1) based on their reliability and the environment. During aging, SR compensates for vestibular decline by increasing reliance on proprioceptive and/ or vision inputs. SR can occur dynamically (eg, in response to changing environmental factors such as poor lighting, moving visual scenes, or uneven or unstable surfaces) or gradually over time due to evolving physiological factors, including vestibular impairments, vision decline, or reduced proprioceptive acuity. Coincidentally, aging also slows SR adaptability, thereby increasing the risk of falls under challenging environmental or physiological conditions.21 Studies have identified vestibular dysfunction as the primary cause of balance decline in more than 55% of adults over age 50,22-25 or approximately 70 million people in the United States. Furthermore, more than 35% of US adults over age 40 already suffer from vestibular deficits,26 often long before symptoms appear. The vestibular system comprises inner ear organs (distinct from those responsible for hearing), the vestibular nerve that transmits motion information to the brain, the vestibular nucleus in the brainstem, and multiple connected brain regions responsible for interpreting and responding to this information. Vestibular sensory information is widely distributed throughout the CNS to support balance and many other physiological and cognitive processes.27 Presbyvestibulopathy can involve dysfunction in both the peripheral vestibular organs in the inner ear and central vestibular processing in the brain.28 Normal aging can lead to loss of motion-sensing vestibular hair cells, decreased gain of the synapses between the hair cells and the vestibular nerve, and reduced excitability of the vestibular nerve. The resulting weakening of the signals received by the brain may also contribute to atrophy in the CNS regions that integrate and process sensory inputs and motor control signals. Gaps in Vestibular Diagnostics and Treatment If vestibular decline is diagnosed, the current standard treatment is vestibular rehabilitation therapy,30 a specialized type of physical therapy that leverages three mechanisms to retrain the brain and body to rebuild a reliable sense of balance: • Habituation normalizes the brain’s reaction to movements or visual environments that trigger dizziness. • Adaptation recalibrates the brain’s response to remaining vestibular function, minimizing discrepancies between vision and inner ear sensation. • Substitution helps patients compensate for weakened vestibular inputs by relying more on vision and proprioception. Vestibular rehabilitation therapy may improve symptoms, but it does not restore degraded vestibular function or prevent further deterioration in motor function. Phybrata: Wearable Balance Diagnostics Previous studies have shown that digital biomarkers derived from phybrata data effectively quantify changes in postural stability, underlying sensory and neuromotor impairments, and the resulting SR due to head trauma,31,33 diseases like multiple sclerosis,34 and spinal cord injuries. 35 The same technology has now been validated for assessing aging-related balance decline.8 Phybrata Assessments of Age-Related Balance Decline The reliability of proprioceptive inputs can also degrade with age due to loss of muscle mass and lower limb strength, peripheral neuropathies, or reduced proprioceptive acuity in the feet and ankles.36,37 As this degraded proprioception becomes the “dominant” system, balance recovery strategies (eg, quick stepping or ankle adjustments) lose effectiveness. Reduced vestibular input and overreliance on proprioception can trigger a spiral of decline in balance and mobility, which also impacts cognitive function and psychological well-being:38-40 Reduced vestibular function → increased reliance on proprioception → poorer balance and gait, especially in challenging environments → reduced mobility and physical activity → deconditioning of gait and postural muscles → further increase in fall risk → fear of falling further reduces balance and mobility confidence → increased cognitive load to support balance → decreased cognitive reservoir for other functions → reduced social activity → decline in cognitive performance and psychological well-being. These findings underscore the critical unmet clinical need for interventions that can help to maintain or restore vestibular function in older adults. Vestibular Stimulation Therapy Leveraging the phybrata sensor and biomarkers, researchers for the first time monitored individual changes in balance performance, SR, and fall risk in response to varying EVS parameters.9 This unique capability led to the discovery of a unique class of EVS waveforms that restore degraded vestibular function (Figure 4B) and trigger significant and long-lasting improvements in balance performance.10 Since these waveforms are imperceptible to users and require no accompanying physical activity, the treatment is comfortable and well-tolerated. Clinical pilots and an initial randomized controlled trial13 with residents of three senior living communities (aged 50 to 95 years) have validated a proprietary vestibular stimulation therapy (VST) treatment protocol63 comprising 18 20-minute treatment sessions over a four- to six-week period. The phybrata-Neurvesta diagnostic-therapeutic combination delivered long-lasting neuroplastic restoration of balance (Figure 4C), accompanied by significant improvements in mobility and gait, as well as a reduction in fall risk (Figure 4D). Significant improvements in balance are typically measured and reported within the initial two treatment sessions. These improvements continue to increase progressively, plateau after 12 to 18 sessions, and then persist for at least six months, at which point a shorter “maintenance” treatment can be administered. Furthermore, phybrata testing can screen out nonresponders (<5% of study participants to date) during the first two treatment sessions, which limits costs and unproductive time for payers, providers, and patients alike. Pilot Deployments of the Neurvesta Device Many pilot participants report immediate improvements in balance and walking within the first two treatment sessions. The two most common improvements are feeling more secure walking down stairs and hiking over uneven terrain, and having an enhanced ability to perform exercises that require standing on one leg. Physical therapists involved in the pilot deployments have noted that the VSTinduced improvements in vestibular control, balance, and mobility enabled patients to progress to more advanced and challenging physiotherapy activities that were previously limited by unstable posture or fear of falling. These mobility gains also extend to cognition (eg, increased dual-task capacity without instability) and psychological well-being, with reduced fear of falling leading to a greater willingness to engage. This represents a significant reversal of the negative spiral discussed earlier. Conclusions — Geoffrey K. Feld, PhD, is founder and principal consultant of Geocyte. — John Ralston, PhD, MBA, is the CEO and founder of Neursantys.
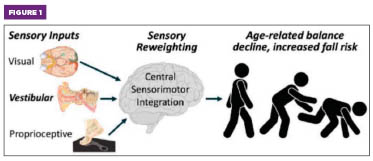
Figure 1. Balance relies on three key sensory inputs. The brain continuously assesses the reliability of these inputs and adjusts their relative utilization through sensory reweighting (SR) to maintain postural stability. Age-related reductions in SR response time or accuracy impair balance and mobility and significantly increase fall risk.
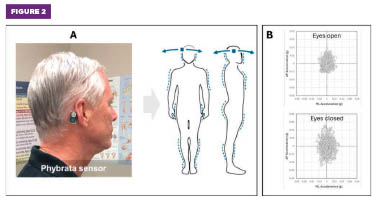
Figure 2. (A) The Phybrata sensor detects and analyzes subtle head and body micromovements during balance and gait tests. (B) Example two-dimensional acceleration data captured during a pair of one-minute eyes open and eyes closed standing balance tests.
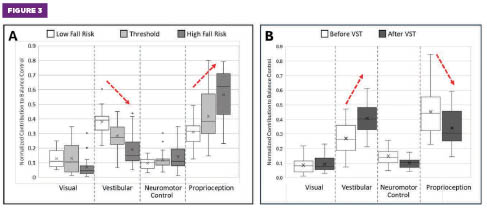
Figure 3. (A) Phybrata sensory reweighting (SR) plots for older adults (N = 516, aged 51 to 98) with increasing balance instability and fall risk reveal a consistent compensatory shift toward proprioceptive balance control as vestibular inputs weaken. (B) Phybrata SR plots for older adults (N = 32, aged 60 to 98) after completing the 18-session vestibular stimulation therapy balance restoration treatment reveal significant restoration of vestibular balance control and reduced reliance on proprioception.
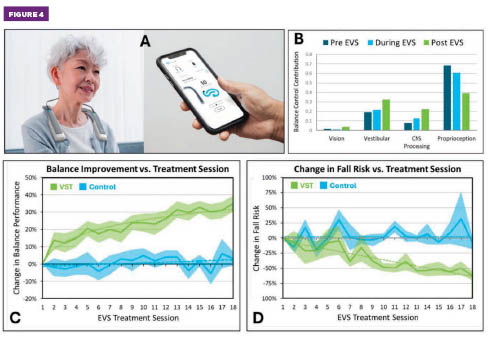
Figure 4. (A) Neurvesta device and mobile app. (B) Example SR measurements pre-, during, and post-electrical vestibular stimulation, revealing enhancement of vestibular function accompanied by decreased reliance on proprioception. (C) Measured balance improvements and (D) reduced fall risk for a randomized controlled trial of the 18-session vestibular stimulation therapy treatment protocol (50 participants, aged 50 to 95). Solid line denotes mean values, and shaded areas represent one standard deviation from the mean. Figures courtesy of Neursantys
References 2. Agrawal Y, Van de Berg R, Wuyts F, Walther L, Magnusson M, Oh E, Sharpe M, Strupp M. Presbyvestibulopathy: Diagnostic criteria Consensus document of the classification committee of the Bárány Society. J Vestib Res. 2019;29(4):161-170. 3. Smith PF. Aging of the vestibular system and its relationship to dementia. Curr Opin Neurol. 2024;37(1):83-87. 4. Smith LJ, Pyke W, Fowler R, Matthes B, de Goederen E, Surenthiran S. Impact and experiences of vestibular disorders and psychological distress: qualitative findings from patients, family members and healthcare professionals. Health Expect. 2024;27(1):e13906. 5. Florence CS, Bergen G, Atherly A, Burns E, Stevens J, Drake C. Medical costs of fatal and nonfatal falls in older adults. J Am Geriatr Soc. 2018;66(4):693-698. 6. Agrawal Y, Pineault KG, Semenov YR. Health-related quality of life and economic burden of vestibular loss in older adults. Laryngoscope Investig Otolaryngol. 2017;3(1):8-15. 7. Guyot J, Perez Fornos A. Milestones in the development of a vestibular implant. Curr Opin Neurol. 2019;32(1):145-153. 8. Ralston JD, Stanley S, Roper JM, Ralston AB. Phybrata digital biomarkers of age-related balance impairments, sensory reweighting, and intrinsic fall risk. Med Devices (Auckl). 2025;18:319-336. 9. Ralston J, Banman C, Peters R. Phybrata-based assessments of sensory reweighting due to postural stability challenges and electrical vestibular stimulation. Paper presented at: Society for the Neural Control of Movement Annual Meeting; 2023. 10. Ralston JD, King JA, Peters RM. Phybrata – novel neuromotor systems biomarkers to quantify normal age-related decline in balance and mobility, accelerated aging caused by disease and trauma, and EVS balance restoration. Paper presented at: 2024 Biomarkers of Aging Symposium; November 1-2, 2024; Harvard Medical School, Boston, Massachusetts. 11. Ralston J, King J, Rempel J, Peters R. Wearable bioelectronic balance restoration in older adults. Paper presented at: AGEWELL Annual Conference; 2023. 12. King J, Banman C, Walters N, Clark S, Ralston J, Peters R. Electrical vestibular stimulation therapeutics for balance and gait in older adults. Paper presented at: Canadian Association on Gerontology 52nd Annual Scientific and Educational Meeting; 2023. 13. King JA, Walters N, Rodrigues N, et al. Electrical vestibular stimulation to improve static balance in older adults: a randomized control trial [published online May 29, 2025]. J Neuroeng Rehabil. doi: 10.21203/rs.3.rs-6349795/v1. 14. Fuentealba M, Rouch L, Guyonnet S, et al. A blood-based epigenetic clock for intrinsic capacity predicts mortality and is associated with clinical, immunological and lifestyle factors. Nat Aging. 2025;5(7):1207-1216. 15. Moqri M, Herzog C, Poganik JR, et al. Validation of biomarkers of aging. Nat Med. 2024;30(2):360-372. 16. Biomarkers of Aging Consortium; Herzog CMS, Goeminne LJE, et al. Challenges and recommendations for the translation of biomarkers of aging. Nat Aging. 2024;4(10):1372-1383. 17. World report on ageing and health. World Health Organization website. https://www.who.int/publications/i/item/9789241565042. Published September 29, 2015. 18. Steindl R, Kunz K, Schrott-Fischer A, Scholtz AW. Effect of age and sex on maturation of sensory systems and balance control. Dev Med Child Neurol. 2006;48(6):477-482. 19. Tian YE, Cropley V, Maier AB, Lautenschlager NT, Breakspear M, Zalesky A. Heterogeneous aging across multiple organ systems and prediction of chronic disease and mortality. Nat Med. 2023;29(5):1221-1231. 20. Assländer L, Peterka RJ. Sensory reweighting dynamics in human postural control. J Neurophysiol. 2014;111(9):1852-1864. 21. Zhang S, Xu W, Zhu Y, Tian E, Kong W. Impaired multisensory integration predisposes the elderly people to fall: a systematic review. Front Neurosci. 2020;14:411. 22. Davis LE. Dizziness in elderly men. J Am Geriatr Soc. 1994;42(11):1184-1188. 23. Iwasaki S, Yamasoba T. Dizziness and imbalance in the elderly: age-related decline in the vestibular system. Aging Dis. 2014;6(1):38-47. 24. Agrawal Y, Merfeld DM, Horak FB, et al. Aging, vestibular function, and balance: proceedings of a National Institute on Aging/National Institute on Deafness and Other Communication Disorders workshop. J Gerontol A Biol Sci Med Sci. 2020;75(12):2471-2480. 25. Wagner AR, Akinsola O, Chaudhari AMW, Bigelow KE, Merfeld DM. Measuring vestibular contributions to age-related balance impairment: a review. Front Neurol. 2021;12:635305. 26. Agrawal Y, Ward BK, Minor LB. Vestibular dysfunction: prevalence, impact and need for targeted treatment. J Vestib Res. 2013;23(3):113-117. 27. Kirsch V, Keeser D, Hergenroeder T, et al. Structural and functional connectivity mapping of the vestibular circuitry from human brainstem to cortex. Brain Struct Funct. 2016;221(3):1291-1308. 28. Agrawal Y, Smith PF, Merfeld DM. Dizziness, imbalance and age-related vestibular loss. The Senses: A Comprehensive Reference. 2020;6:567-580. 29. Wagner AR, Akinsola O, Chaudhari AMW, Bigelow KE, Merfeld DM. Measuring vestibular contributions to age-related balance impairment: a review. Front Neurol. 2021;12:635305. 30. Deveze A, Bernard-Demanze L, Xavier F, Lavieille JP, Elziere M. Vestibular compensation and vestibular rehabilitation. Current concepts and new trends. Neurophysiol Clin. 2014;44(1):49-57. 31. Ralston JD, Raina A, Benson BW, Peters RM, Roper JM, Ralston AB. Physiological vibration acceleration (Phybrata) sensor assessment of multi-system physiological impairments and sensory reweighting following concussion. Med Dev (Auckl). 2020;13:411-438. 32. Lin IS, Lai DM, Ding JJ, et al. Reweighting of the sensory inputs for postural control in patients with cervical spondylotic myelopathy after surgery. J Neuroeng Rehabil. 2019;16(1):96. 33. Hope AJ, Vashisth U, Parker MJ, Ralston AB, Roper JM, Ralston JD. Phybrata sensors and machine learning for enhanced neurophysiological diagnosis and treatment. Sensors (Basel). 2021;21(21):7417. 34. Heydari S, Lacey C, Stewart-Good K, Mayo C, Ralston J, Gawryluk J. The relationship between phybrata wearable sensor data and white matter microstructure in people with multiple sclerosis. Paper presented at: Consortium of Multiple Sclerosis Centers Annual Meeting; 2023. 35. Lei Y, Rios V, Ji J, Duhon B, Boyd H, Xu Y. Quantifying unsupported sitting posture impairments in humans with cervical spinal cord injury using a head-mounted IMU sensor. Spinal Cord. 2024;62(2):65-70. 36. Pasma JH, Engelhart D, Maier AB, Schouten AC, van der Kooij H, Meskers CGM. Changes in sensory reweighting of proprioceptive information during standing balance with age and disease. J Neurophysiol. 2015;114(6):3220-3233. 37. Yamazaki K, Sakai Y, Ito T, Fukuhara J, Morita Y. Percentage of decline in individual proprioceptors in older adults. J Phys Ther Sci. 2024;36(9):492-497. 38. Wang J, Li Y, Yang GY, Jin K. Age-related dysfunction in balance: a comprehensive review of causes, consequences, and interventions. Aging Dis. 2024;16(2):714-737. 39. Guo J, Wang J, Liang P, et al. Vestibular dysfunction leads to cognitive impairments: state of knowledge in the field and clinical perspectives (review). Int J Mol Med. 2024;53(4):36. 40. Smith LJ, Wilkinson D, Bodani M, Surenthiran SS. Cognition in vestibular disorders: state of the field, challenges, and priorities for the future. Front Neurol. 2024;15:1159174. 41. Neursantys NEURVESTA. Cortex Design website. https://cortex-design.com/work/neursantys/ 42. Steadman CJ, Abd-El Barr MM, Lad SP, Gad P, Gorgey AS, Hoenig H. Bioelectric medicine: electrotherapy and transcutaneous electromagnetic stimulation-clinical and research challenges. Arch Phys Med Rehabil. 2022;103(11): 2268-2271. 43. Jung B, Yang C, Lee SH. Electroceutical and bioelectric therapy: its advantages and limitations. Clin Psychopharmacol Neurosci. 2023;21(1):19-31. 44. Field-Eaton C, Pellumbi G. Bioelectronics ‘jump-start’ the next wave of device therapeutics. McKinsey & Company website. https://www.mckinsey.com/industries/life-sciences/our-insights/bioelectronics-jump-start-the-next-wave-of-device-therapeutics. Published October 3, 2019. 45. Lopez C, Cullen KE, Electrical stimulation of the peripheral and central vestibular system. Curr Opin Neurol. 2024;37(1):40-51. 46. Dlugaiczyk J, Gensberger KD, Straka H. Galvanic vestibular stimulation: from basic concepts to clinical applications. J Neurophysiol. 2019;121(6):2237-2255. 47. Marchand S, Langlade A, Legois Q, Séverac Cauquil A. A wide-ranging review of galvanic vestibular stimulation: from its genesis to basic science and clinical applications. Exp Brain Res. 2025:243(5):131. 48. Pires APBÁ, Silva TR, Torres MS, Diniz ML, Tavares MC, Gonçalves DU. Galvanic vestibular stimulation and its applications: a systematic review. Braz J Otorhinolaryngol. 2022;88 Suppl 3(Suppl 3):S202-S211. 49. Fu W, Bai Y, Wang X. Galvanic vestibular stimulation for postural rehabilitation in neurological disorders: a systematic review. Front Neurosci. 2025;19:1580078. 50. Lajoie K, Marigold DS, Valdés BA, Menon C. The potential of noisy galvanic vestibular stimulation for optimizing and assisting human performance. Neuropsychologia. 2021;152:107751. 51. White O, Babic J, Trenado C, Johannsen L, Goswami N. The promise of stochastic resonance in falls prevention. Front Physiol. 2019;9:1865. 52. Herssens N, McCrum C. Stimulating balance: recent advances in vestibular stimulation for balance and gait. J Neurophysiol. 2019;122(2):447-450. 53. Wuehr M, Decker J, Schniepp R. Noisy galvanic vestibular stimulation: an emerging treatment option for bilateral vestibulopathy. J Neurol. 2017;264(Suppl 1):81-86. 54. Haxby F, Akrami M, Zamani R. Finding a balance: a systematic review of the biomechanical effects of vestibular prostheses on stability in humans. J Funct Morphol Kinesiol. 2020;5(2):23. 55. Kwan A, Forbes PA, Mitchell DE, Blouin JS, Cullen KE. Neural substrates, dynamics and thresholds of galvanic vestibular stimulation in the behaving primate. Nat Commun. 2019;10(1):1904. 56. Gensberger KD, Kaufmann AK, Dietrich H, et al. Galvanic vestibular stimulation: cellular substrates and response patterns of neurons in the vestibulo-ocular network. J Neurosci. 2016;36(35):9097-9110. 57. Huang Y, Mao H, Chen Y. Regeneration of hair cells in the human vestibular system. Front Mol Neurosci. 2022;15:854635. 58. Mitsutake T, Sakamoto M, Kawaguchi A, Tamari M, Horikawa E. Greater functional activation during galvanic vestibular stimulation is associated with improved postural stability: a GVS-fMRI study. Somatosens Mot Res. 2020;37(4):257-261. 59. Fujimoto C, Yamamoto Y, Kamogashira T, et al. Noisy galvanic vestibular stimulation induces a sustained improvement in body balance in elderly adults. Sci Rep. 2016;6:37575, 60. Inukai Y, Masaki M, Otsuru N, et al. Effect of noisy galvanic vestibular stimulation in community-dwelling elderly people: a randomised controlled trial. J Neuroeng Rehabil. 2018;15(1):63, 61. Serrador JM, Deegan BM, Geraghty MC, Wood SJ. Enhancing vestibular function in the elderly with imperceptible electrical stimulation. Sci Rep. 2018;8(1):336. 62. Jain A, Sarkar A, Gupta M. Optimizing balance using vestibular electrical stimulation to study its therapeutic effect among elderly. Bioscience Biotechnology Research Communications. 2022;15(2):313-320. 63. Therapeutic effects of electrical vestibular stimulation (EVS) on balance and gait (VST). ClinicalTrials.gov website. https://clinicaltrials.gov/study/NCT06846047?id=NCT06846047&rank=1. Updated February 25, 2025. 64. Neursantys and Caring Hands Caregivers partner to pilot bioelectronic balance restoration for senior living communities. MedTech Dive website. https://www.medtechdive.com/press-release/20250312-neursantys-and-caring-hands-caregivers-partner-to-pilot-bioelectronic-balan/. Published March 12, 2025. 65. Araujo CG, de Souza E Silva CG, Laukkanen JA, et al. Successful 10-second one-legged stance performance predicts survival in middle-aged and older individuals. Br J Sports Med. 2022;56(17):975-980. 66. Anson E, Bigelow RT, Studenski S, Deshpande N, Agrawal Y. Failure on the foam eyes closed test of standing balance associated with reduced semicircular canal function in healthy older adults. Ear Hear. 2019;40(2):340-344. |