March/April 2020
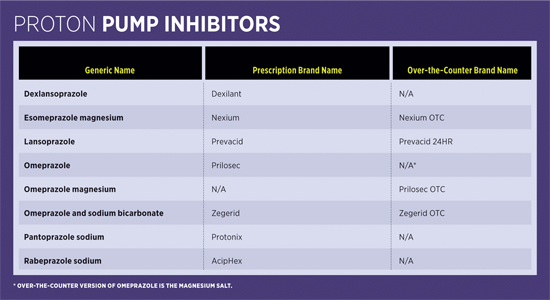
Medication Monitor: The Dark Side of Proton Pump Inhibitors Despite being effective for gastrointestinal disorders, these medications are associated with significant risks and increased mortality. Proton pump inhibitors (PPIs) are frequently used to treat gastrointestinal (GI) disorders such as dyspepsia, gastroesophageal reflux disease, erosive esophagitis, and NSAID-induced ulcers. The acid-suppressing medications are among the most commonly used classes of medication in the United States—an estimated 7.8% of the adult population take prescription PPIs.1 The proven efficacy and ease of availability of prescription and over-the-counter (OTC) PPI products (see table) have contributed to their widespread use and, possibly, the common belief these medications can be used with minimal safety concerns. However, over the past decade there’s been mounting evidence that PPI use may be associated with significant risk, including an elevated risk of acute kidney injury, chronic kidney disease (CKD), bone fractures, infections, possible dementia, and increased mortality.
Concerns About Inappropriate Use With extended use, efforts to discontinue PPIs can be complicated due to rebound hypersecretion, which can occur when the body’s normal gastric acid has been suppressed and PPI use ends. In 60% to 90% of patients taking PPIs for at least two to three months, increased gastric acid secretion can occur when the PPI is stopped.6 For these patients, a step-down approach to another GI medication such as a H2 blocker or slowly reducing the dose can help with efforts to discontinue the PPI. Nutritional and Infection Risk Clostridium difficile–Associated Diarrhea (CDAD) Patients taking PPIs have been shown to have 1.4 to 2.75 times greater risk of developing CDAD than do patients not receiving PPI therapy. CDAD can also be especially concerning in hospitals, with 1 in 533 hospitalized patients taking PPIs developing CDAD. One study found that patients with no recent hospitalization have an increased CDAD infection risk after only two days of PPI use, while patients with a hospital admission within the previous 30 days have an increased risk after only one day of therapy.8 Pneumonia In one study, 75,000 adults older than 60 who had taken prescribed PPIs regularly over a two-year period were shown to have a higher rate of pneumonia compared with those who did not take PPIs.10 The increased risk of pneumonia may be associated with the reflux of gastric contents along with bacteria in the esophagus. Gastroenteritis Osteoporosis and Fracture Risk PPIs can also reduce the effectiveness of bisphosphonates (eg, alendronate), which are used to help reduce bone loss from osteoporosis and prevent hip fractures. Patients older than 70 taking PPIs with the bisphosphonate alendronate experienced a significantly increased fracture risk, and when PPI therapy was given together with alendronate for two years or more, the potential hip fracture protection was negated.12 Hypomagnesemia Renal Concerns A large observational study using data from the Atherosclerosis Risk in Communities cohort and the Geisinger Health System found that use of PPIs increased the risks of CKD, CKD progression, and end-stage renal failure. According to the study, more than one-half of those who went on to experience kidney damage had no prior history of acute kidney injury before taking the drugs.15 Dementia Mortality Risk Recommendations • Increase patient awareness about the possible risks associated with PPIs. • Actively look for opportunities to deprescribe PPIs. • Recommend using the lowest possible doses for the shortest possible duration. • Limit long-term PPI use to persons requiring routine NSAIDs and those with severe esophagitis, Barrett’s esophagus, gastroesophageal reflux disease, Zollinger-Ellison syndrome, and bleeding ulcers. — Mark D. Coggins, PharmD, BCGP, FASCP, is vice president of pharmacy services and medication management for skilled nursing centers operated by Diversicare in nine states and is a past director on the board of the American Society of Consultant Pharmacists. He was nationally recognized by the Commission for Certification in Geriatric Pharmacy with the 2010 Excellence in Geriatric Pharmacy Practice Award.
References 2. Katz MH. Failing the acid test: benefits of proton pump inhibitors may not justify the risks for many users. Arch Intern Med. 2010;170(9):747-748. 3. Hamzat H, Sun H, Ford JC, Macleod J, Soiza RL, Mangoni AA. Inappropriate prescribing of proton pump inhibitors in older patients: effects of an educational strategy. Drugs Aging. 2012;29(8):681-690. 4. Pandolfino J. Discontinuation of proton pump inhibitor therapy and the role of esophageal testing. Gastroenterol Hepatol (N Y). 2013;9(11):747-764. 5. Rotman SR, Bishop TF. Proton pump inhibitor use in the U.S. ambulatory setting, 2002-2009. PLoS One. 2013;8(2):e56060. 6. Osefo N, Ito T, Jensen RT. Gastric acid hypersecretory states: recent insights and advances. Curr Gastroenterol Rep. 2009;11(6):433-441. 7. FDA drug safety communication: Clostridium difficile associated diarrhea can be associated with stomach acid drugs known as proton pump inhibitors (PPIs). FDA website. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-clostridium-difficile-associated-diarrhea-can-be-associated-stomach. Updated May 9, 2017. 8. Dial SM. Proton pump inhibitor use and enteric infections. Am J Gastroenterol. 2009;104 Suppl 2:S10-S16. 9. Fohl AL, Regal RE. Proton pump inhibitor-associated pneumonia: not a breath of fresh air after all? World J Gastrointest Pharmacol Ther. 2011;2(3):17-26. 10. Zirk-Sadowski J, Masoli JA, Delgado J, et al. Proton-pump inhibitors and long-term risk of community-acquired pneumonia in older adults. J Am Geriatr Soc. 2018;66(7):1332-1338. 11. Vilcu A, Sabatte L, Blanchon T, et al. Association between acute gastroenteritis and continuous use of proton pump inhibitors during winter periods of highest circulation of enteric viruses. JAMA Netw Open. 2019;2(11):e1916205. 12. Abrahamsen B, Eiken P, Eastell R. Proton pump inhibitor use and the antifracture efficacy of alendronate. Arch Intern Med. 2011;171(11):998-1004. 13. Florentin M, Elisaf MS. Proton pump inhibitor-induced hypomagnesemia: a new challenge. World J Nephrol. 2012;1(6):151-154. 14. Antoniou T, Macdonald EM, Hollands S, et al. Proton pump inhibitors and the risk of acute kidney injury in older patients: a population-based cohort study. CMAJ Open. 2015;3(2):E166-E171. 15. Wyatt CM. Proton pump inhibitors and chronic kidney disease: is it time to sound the alarm? Kidney Int. 2016;89(4):732-733. 16. Wod M, Hallas J, Andersen K, García Rodríguez LA, Christensen K, Gaist D. Lack of association between proton pump inhibitor use and cognitive decline. Clin Gastroenterol Hepatol. 2018;16(5):681-689. 17. Xie Y, Bowe B, Yan Y, Xian H, Li T, Al-Aly Z. Estimates of all cause mortality and cause specific mortality associated with proton pump inhibitors among US veterans: cohort study. BMJ. 2019;365:l1580. |