November/December 2021
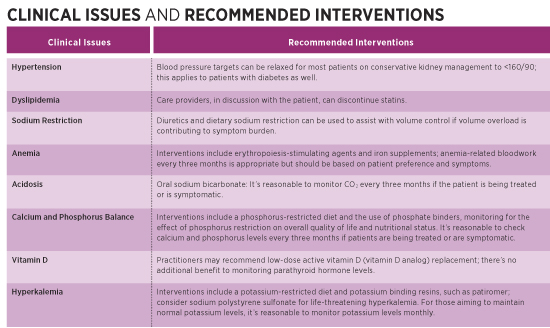
Geriatric Kidney Disease Harnessing Evidence-Based Medicine to Improve Outcomes and Quality of Life Chronic kidney disease (CKD) and end-stage renal disease (ESRD) are serious medical conditions and major drivers of health care spending. According to a report from the US Department of Health and Human Services, Medicare spending for beneficiaries with CKD and ESRD was more than $130 billion in 2018, representing more than 20% of total Medicare fee-for-service expenditures. At the same time, health outcomes for patients with CKD and ESRD are poor, with evidence showing that outcomes for kidney failure are even worse than are those for other serious medical conditions such as cancer and congestive heart failure.1 This article discusses an evidence-based approach to care that geriatricians—in partnership with primary care physicians and nephrologists—can and should adopt for patients with CKD and ESRD. It explores the advantages of conservative kidney management (CKM), a model that focuses on minimizing suffering, better managing co-occurring conditions and symptoms, educating and empowering patients, and supporting their decisions regarding appropriate interventions—including alternatives to hemodialysis—to maximize outcomes and quality of life. Geriatric patients already experience many of the same issues as do patients with late-stage CKD and ESRD—multiple chronic diseases, polypharmacy, and a high burden of psychosocial issues, all coming at the end of life. Increasingly, older patients with kidney disease are now facing the complex decision about how to best manage their failing kidneys. Older patients comprise the fastest-growing population initiating dialysis, and many have coexisting conditions that affect their experience and survival.1 These dialysis patients spend their last days undergoing intensive therapies at a higher rate than do those with other life-limiting conditions and have higher rates of hospitalization and a greater likelihood of dying in the hospital.2 In the United States, dialysis is often viewed as the default (if not only) treatment for advanced kidney disease, and it’s relatively uncommon for patients who develop the signs and symptoms of advanced kidney disease to avoid dialysis and its burdens. In other developed countries, far fewer older adults are initiated on dialysis, suggesting dialysis isn’t always the best treatment option for kidney failure.1 Sometimes the best course of care is to manage late-stage kidney disease with a conservative approach. By applying the principles of geriatric medicine—which involve consideration of psychosocial issues, involvement of multiple disciplines, and, above all, respect for patient autonomy—clinicians can better support older adults living with advanced kidney disease. Conservative Care vs Dialysis Although dialysis is commonly regarded as life-prolonging therapy for patients with advanced CKD, it doesn’t always achieve the intended effect of lengthening life and restoring health and quality of life.3 CKM is increasingly recognized as an acceptable and beneficial treatment option as an alternative to dialysis. For older adults with a high burden of comorbid conditions, especially ischemic heart disease and functional or cognitive impairment, whether they start dialysis or are managed conservatively makes little difference for life expectancy but can have a significant impact on quality of life.4 Patients who forego dialysis spend less time in health care settings and are less likely to undergo invasive procedures and to die in the hospital.5,6 In addition, patients who start dialysis can have significant functional decline, resulting in the need for increased caregiver support or long-term care.7 Comparisons have been made between the morbidity and mortality of late-stage kidney disease and advanced cancer, where a palliative approach to care is widely accepted. Since the evidence generally shows that the older the patient, the worse the outcomes will be on dialysis, CKM similarly should be accepted as an integral part of renal care.8 In determining whether to initiate dialysis, primary care physicians and nephrologists should have a frank, prognosis-based discussion that addresses balancing the benefits and risks of dialysis treatment. Attending dialysis three or more times per week requires lifestyle changes that impose limits on independence, along with dietary restrictions that may interfere with usual daily activities. Complications associated with dialysis include postdialysis fatigue, intradialytic and postdialysis hypotension, headache, and cramping. Dialysis patients spend more time in the hospital with the complications of treatment of comorbid conditions.5 Patients are at increased risk of sepsis and often require interventions related to vascular access. Patient Identification Even in the outpatient setting, clinicians often present dialysis to the patient as a necessity rather than a choice: “If you don’t do dialysis, you will die.” Clinicians often share minimal or no information on the alternative to dialysis—CKM—because CKM hasn’t yet gained widespread understanding and acceptance. Even nephrologists who have many patients with significant disease burden may have limited knowledge about CKM and end-of-life care.10 Ideally, discussion of a palliative approach to care should occur with all patients with life-limiting illnesses, including CKD stages 4 and 5. This approach to care focuses on what matters most to patients and relies on the four pillars of palliative care: patient identification, advance care planning, symptom assessment and management, and care of the dying patient. Specific interventions focus on active medical management without dialysis. Discussing treatment options with an individual who’s unlikely to benefit from dialysis can be challenging. It’s important to discuss with patients the risks of dialysis and explain that while dialysis may prolong their lives, it may not improve their quality of life.4,5,11 The “extra” time afforded by dialysis may be spent in the dialysis chair or the hospital. Discussions that involve end-of-life care are quite understandably fraught with emotion, specific family dynamics, and other complications. Sometimes it’s the patient’s family who requests interventions, and not necessarily the patient. Frequently, they say, “We want everything done.” Yet respect for the patient’s autonomy requires that mentally capable older adults be allowed to make informed treatment decisions without undue influence from others. Additionally, physicians, in driving a prognosis-based decision and respecting the patient’s wishes, can say, “Everything has been done.” The key concept is that the patient makes an informed choice. CKM should be considered “active supportive care” and doesn’t equate to “doing nothing” or “giving up.” The basics of CKM include the following12,13: • interventions to delay progression of kidney disease and minimize risk of adverse events or complications; Conservative Interventions
Geriatric Considerations The incidence of dementia increases with age, and, as with renal disease, hypertension is a risk factor for vascular dementia. Dementia is a progressive disease that results in unrelenting cognitive and functional decline. The percentage of people with Alzheimer’s dementia increases with age: 3% of people aged 65 to 74, 17% of people aged 75 to 84, and 32% of people aged 85 and older have Alzheimer’s dementia.15 Dementia is a predictor of mortality in patients on dialysis.16 A substantial portion of those who would meet the diagnostic criteria for Alzheimer’s and other dementias aren’t diagnosed with dementia by a physician.17 Preservation of social skills in early dementia make it not easily recognizable during brief physician encounters. In addition, 25% of hospitalized patients have dementia that’s unrecognized and undocumented.18 Patients with dementia may not be able to consent to dialysis on an informed basis, understand the risks and benefits, or even be able to sit through a dialysis treatment. Functional decline and physical dependence are common in old age. Functional status—the ability to perform activities of daily living such as bathing, dressing, transferring, feeding, ambulation, and toileting—is a key component of quality of life, a strong predictor of survival, a determinant of caregiving needs and health care costs, and a factor in decisions about medical procedures.19 Functional disability has been defined as acquired difficulty in performing basic everyday tasks or more complex tasks needed for independent living. Unfortunately, assessment of an older person’s function isn’t a common practice, and there’s increased all-cause mortality associated with functional disability.20 Loss of independence in patients starting dialysis at 80 years or older is common. In one study of patients older than the age of 80, at time of dialysis the majority were living at home with no assistance for ADLs.21 Within the first six months, more than 30% had functional loss requiring community or private-caregiver support or transfer to a nursing home. This rate of functional loss is higher than that of the 20% of frail, nondialysis elderly patients who have a disability or require nursing home care within 18 months of a hospital discharge.22 This is even more common in nursing home residents, more than one-half of whom had died at 12 months after dialysis. Frailty is a clinically recognizable, aging-related syndrome of physiological decline and decreased functional reserve characterized by marked vulnerability to adverse health outcomes.23 Common hallmarks of frailty include functional decline, falls, sarcopenia, and weight loss. Frail older patients often present with an increased burden of symptoms, including weakness and fatigue, medical complexity, and reduced tolerance to medical and surgical interventions. Frailty is common in all stages of chronic kidney disease; its prevalence increases with decreasing glomerular filtration rate and has been linked with adverse outcomes and increased risk of mortality and hospitalization.24-26 The severity of a patient’s frailty should be taken into consideration when discussing treatment options as the patient approaches end-stage renal disease. Goal-setting with patients and their families is crucial in providing care for the frail individual, establishing individual priorities, weighing risks and benefits of interventions, and making decisions regarding aggressiveness of care. End-of-life planning is an inevitable part of caring for geriatric patients. Given the high mortality of dialysis patients, it’s surprising that less than one-third of dialysis patients complete advance directives.27 Without an advance directive, the default becomes intensive care patterns including multiple hospital admissions, surgical procedures, and resuscitation attempts at the end of life. This cycle of suffering results from a lack of understanding among both patients and their physicians about the morbidity and mortality associated with ESRD and the lack of consideration of CKM for those whose kidneys are failing. Conclusion Geriatricians, with their experience in managing older adults with life-limiting illnesses, functional disability, frailty, and cognitive impairment can lead the way in changing the dialysis paradigm for older patients with kidney disease. They can start by informing their patients about CKM as a reasonable alternative to dialysis. — Deborah Robin, MD, MHCM, is senior vice president, geriatrics and medical director at Monogram Health. An experienced geriatrician and physician executive, she’s held clinical and administrative roles in acute, postacute, and corporate settings. She holds a medical degree from the State University of New York Upstate Medical University and a master’s degree in health care management from Harvard University.
References 2. Wong SPY, Kreuter W, O’Hare AM. Treatment intensity at the end of life in older adults receiving long-term dialysis. Arch Intern Med. 2012;172(8):661-663. 3. Murtagh FEM, Marsh JE, Donohoe P, Ekbal NJ, Sheerin NS, Harris FE. Dialysis or not? A comparative survival study of patients over 75 years with chronic kidney disease stage 5. Nephrol Dial Transplant. 2007;22(4):1955-1962. 4. Da Silva-Gane M, Wellsted D, Greenshields H, Norton S, Chandna SM, Farrington K. Quality of life and survival in patients with advanced kidney failure managed conservatively or by dialysis. Clin J Am Soc Nephrol. 2012;7(12):2002-2009. 5. Carson RC, Juszczak M, Davenport A, Burns A. Is maximum conservative management an equivalent treatment option to dialysis for elderly patients with significant comorbid disease? Clin J Am Soc Nephrol. 2009;4(10):1611-1619. 6. Smith C, Da Silva-Gane M, Chandna S, Warwicker P, Greenwood R, Farrington K. Choosing not to dialyse: evaluation of planned non-dialytic management in a cohort of patients with end-stage renal failure. Nephron Clin Pract. 2003;95(2):c40-c46. 7. Jassal SV, Watson D. Dialysis in late life: benefit or burden. Clin J Am Soc Nephrol. 2009;4(12):2008-2012. 8. Wachterman MW, O’Hare AM, Rahman OK, et al. One-year mortality after dialysis initiation among older adults. JAMA Intern Med. 2019;179(7):987-990. 9. Wong SPY, Kreuter W, O’Hare AM. Healthcare intensity at initiation of chronic dialysis among older adults. J Am Soc Nephrol. 2014;25(1):143-149. 10. Wong SPY, McFarland LV, Liu C-F, Laundry RJ, Herbert PL, O’Hare AM. Care practices for patients with advanced kidney disease who forgo maintenance dialysis. JAMA Intern Med. 2019;179(3):305-313. 11. Seow YY, Cheung YB, Qu LM, Yee ACP. Trajectory of quality of life for poor prognosis stage 5D chronic kidney disease with and without dialysis. Am J Nephrol. 2013;37(3):231-238. 12. Davison SN, Tupala B, Wasylynuk BA, Siu V, Sinnarajah A, Triscott J. Recommendations for the care of patients receiving conservative kidney management. Clin J Am Soc Nephrol. 2019;14(4):626-634. 13. Davison SN, Levin A, Moss AH, et al. Executive summary of the KDIGO Controversies Conference on Supportive Care in Chronic Kidney Disease: developing a roadmap to improving quality care. Kidney Int. 2015;88(3):447-459. 14. Murtagh FEM, Sheerin NS, Addington-Hall J, Higginson IJ. Trajectories of illness in stage 5 chronic kidney disease: a longitudinal study of patient symptoms and concerns in the last year of life. Clin J Am Soc Nephrol. 2011;6(7):1580-1590. 15. 2020 Alzheimer’s disease facts and figures. Alzheimers Dement. 2020;16(3):391-460. 16. Rakowski DA, Caillard S, Agodoa LY, Abbott KC. Dementia as a predictor of mortality in dialysis patients. Clin J Am Soc Nephrol. 2006;1(5):1000-1005. 17. Bradford A, Kunik ME, Schulz P, Williams SP, Singh H. Missed and delayed diagnosis of dementia in primary care: prevalence and contributing factors. Alzheimer Dis Assoc Disord. 2009;23(4):306-314. 18. Mezey M, Maslow K. Recognition of dementia in hospitalized older adults. Hartford Institute for Geriatric Nursing website. https://hign.org/consultgeri/try-this-series/recognition-dementia-hospitalized-older-adults. Published 2016. 19. Wu LW, Chen W-L, Peng TC, et al. All-cause mortality risk in elderly individuals with disabilities: a retrospective observational study. BMJ Open. 2016;6(9):e011164. 20. Inouye SK, Peduzzi PN, Robison JT, Hughes JS, Horwitz RI, Concato J. Importance of functional measures in predicting mortality among older hospitalized patients. JAMA. 1998;279(15):1187-1193. 21. Jassal SV, Chiu E, Hladunewich M. Loss of independence in patients starting dialysis at 80 years of age or older. N Engl J Med. 2009;361(16):1612-1613. 22. Kurella Tamura M, Covinsky KE, Chertow GM, Yaffe K, Landefield CS, McCulloch CE. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med. 2009;361(16):1539-1547. 23. Xue Q-L. The frailty syndrome: definition and natural history. Clin Geriatr Med. 2011;27(1):1-15. 24. Nixon AC, Bampouras TM, Pendleton N, Woywodt A, Mitra S, Dhaygude A. Frailty and chronic kidney disease: current evidence and continuing uncertainties. Clin Kidney J. 2018;11(2):236-245. 25. Bao Y, Dalrymple L, Chertow GM, Kaysen GA, Johansen KL. Frailty, dialysis initiation, and mortality in end-stage renal disease. Arch Intern Med. 2012;172(14):1071-1077. 26. Chowdhury R, Peel NM, Krosch M, Hubbard RE. Frailty and chronic kidney disease: a systematic review. Arch Gerontol Geriatr. 2017;68:135-142. 27. Davison SN. Faqcilitating advance care planning for patients with end-stage renal disease: the patient perspective. Clin J Am Soc Nephrol. 2006;1(5):1023-1028. |